A clinical trial has shown the success of stem cell therapy in reversing corneal damage that can lead to blindness.
According to RCO News Agency, Eye injuries that damage the cornea are usually irreversible and lead to blindness, but a new clinical trial has repaired the damage in patients thanks to the transplantation of stem cells from their healthy eye.
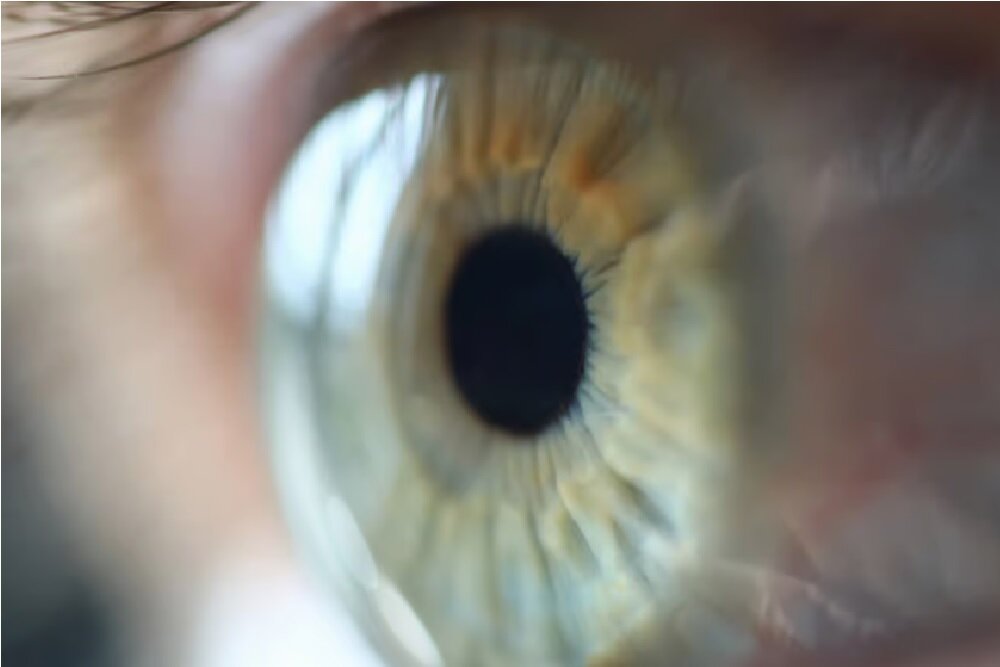
According to the New Atlas, the cornea is the outer layer of the eye that focuses the light towards the retina. Since the cornea is at the forefront of the potential dangers of the outside world, it has a population of Limbal Epitelial stem cells that repair minor damage to keep the cornea surface smooth and functional.
Unfortunately, damage such as thermal or chemical burns can damage the cornea more than the ability of these static stem cells, and little can be done. Even if the damage is very severe, the corneal transplant is not done.
A new study conducted by eye and ear scientists by Massachusetts, examined a new treatment called Ethologic Limbal cells (Calec), which included removing stem cells from the patient’s healthy eye, their population growth in the laboratory for several weeks, and then surgical transplantation.
This clinical trial selected 14 patients to do this treatment and followed them for 18 months. Success was primarily evaluated by how the corneal level was effectively repaired, while a secondary experiment analyzed the improvement in vision.
With the first examination in three months after treatment, the cornea was fully restored to 7 people (50 %). At the end of 12 months, this number increased to 11 (79 %). The other two participants were relatively successful. Therefore, the researchers claim that the overall success rate of their new = treatment is 92 %.
It is worth noting that three participants needed the second transplantation of stem cells, one of which reached full success by the end of the study. In experiments, visual levels in the damaged eye, most patients gained vision and some of the complete blindness came to the point.
No serious side effects attributed to this method were not observed in patients, whether in the healthy and donor eye or in the recipient eye.
Researchers say this success paves the way for more tests with larger groups and longer follow -up before providing the Calec treatment to approve to the US Food and Drug Administration (FDA).
This research is published in the journal Nature Communications.
The end of the message
(tagstotranslate) cornea (T) Stem cell transplantation
RCO NEWS