Although changes to brain neurons, including cell loss, may begin early in life, a drug long approved for other diseases could be repurposed to slow this damage, university scientists have discovered. A topic that creates new hope for people with Alzheimer’s disease and other cognitive disorders.
According to RCO News Agency, Sargramostim, also known as Leukine, is a synthetic form of the natural human protein GM-CSF and has been used for 30 years to treat a variety of diseases, including cancer. This drug has also shown promising results in its first clinical trial by improving blood biomarkers related to brain pathology. The improvement in these markers lasted only as long as the drug was taken, but the memory improvement in one index lasted longer.
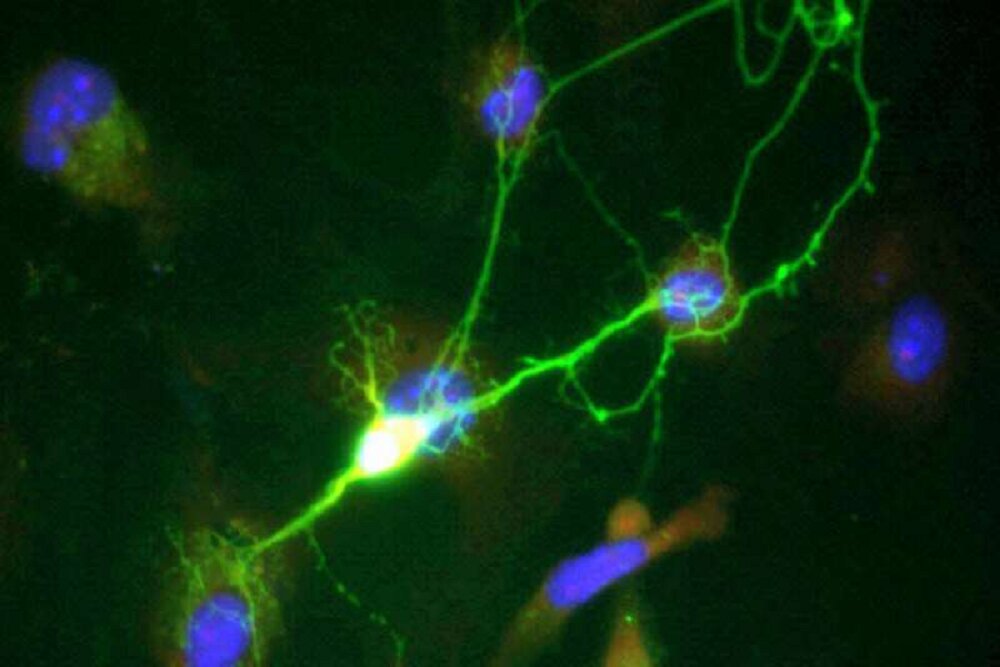
According to Medical Express, the new study in a cross-sectional survey of people of all ages showed that a protein called UCH-L1, which enters the blood from dying neurons in the brain, and another protein called NfL, which is released from damaged neurons, are present in the blood in low concentrations early in life, but their levels increase exponentially every year until the age of 85.
Early changes of this biomarker in early life are probably a reflection of the natural aging process, but in later stages of life, increased levels of UCH-L1 are associated with other outcomes. The discovery could lead to earlier tests and new treatments for Alzheimer’s and possibly cognitive decline caused by natural aging.
The researchers also found that the concentration of GFAP protein, which is an indicator of brain inflammation and is thought to play a key role in promoting cognitive decline, increases significantly in the blood from the age of 40. Interestingly, age-related blood concentrations of GFAP and UCH-L1 are higher in women; The reasons are still unclear.
How might sargramostim work?
The natural protein GM-CSF stimulates the immune system, causing new immune cells to be produced in the bone marrow and brain, while simultaneously regulating inflammation. Sargramostim treatment also improved the scores of one of the cognitive tests compared to the placebo group.
Whether or not this drug can reduce the neuronal damage associated with Alzheimer’s is still unclear and requires further studies. 45 days after the end of the treatment, the blood concentration of UCH-L1 returned to the pre-treatment level, but the cognitive index improvement was maintained. Also, more research is needed to determine whether this drug can reduce neuron death and cognitive decline associated with natural aging.
The authors note that the results of this study are still preliminary and changes in neuronal blood markers are expected as a natural part of the aging process throughout life. A second, longer and more extensive clinical trial of the drug is currently underway in patients with mild to moderate Alzheimer’s. Until clinical studies are completed and the US Food and Drug Administration (FDA) reviews the data and approves sargramostim for the treatment of Alzheimer’s, this drug should not be prescribed or used for any unapproved use.
end of message
RCO NEWS