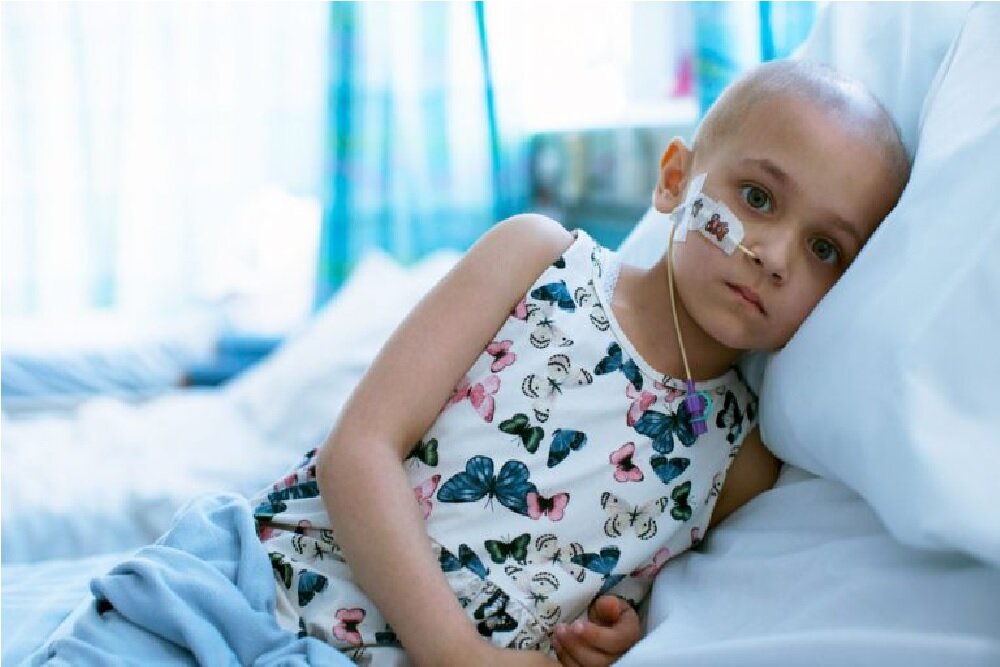
According to scientists, cancer in humans and dogs is very similar, which helps to develop effective treatments for humans.
According to RCO News Agency, Positive results from clinical tests have been achieved an immune treatment in dogs with bone cancer to build a drug to treat children with bone cancer. This shows how the use of genetic similarity between humans and dogs can be useful for both species.
According to the New Atlantic, the field is called “comparative oncology” and is the study of natural cancers in animals such as dogs and cats as a model for the treatment of human diseases.
Animal cancers that appear spontaneously have common properties with human cancers, including bone cancer, prostate and breast cancer, non -Huchkin lymphoma and melanoma.
New York -based OS Therapies Biotechnology focuses on the development of immune therapy for bone cancer and other solid tumors and uses “comparative cancer” to achieve its goal.
The company has recently announced that a subsidiary, OS Animal Health, has founded a treatment called OST-HER2 to treat dogs for bone cancer treatment using clinical trial data for quick tracking for children with this type of cancer.
“Bone cancer is the most common cancer among dogs that affects more than 40,000 dogs in the United States every year,” says Paul Romness, CEO and head of the company. We now now have a clear business opportunity to dramatically improve health results in this deadly cancer through “OS-HER2” commercial production process.
HER2 treatments target cancers exposed to the growth factor of the epidermis 2 (HER2), which include breast, esophageal, lung, ovarian, pancreatic cancers as well as bone cancers. These cancers produce high levels of HER2 protein that accelerate the growth and expansion of the tumor.
Treatment of “Ost-HER2” using a modified Listeria Monocytogenes bacteria to provide DNA therapy to cells stimulates the strong immune system response and causes T cells to target protein.
While bone cancer can appear in any bone in the body of the dogs, it usually appears in the bones of the body weight, which is invasive and malignant and often expands to the lungs.
Tumor removal and sometimes extinction is a common treatment of this cancer, while chemotherapy with surgery is also performed to improve survival time by reducing the risk of metastasis.
The recent clinical trial of Ost-Her2 treatment by OS Therapies in dogs with bone cancer published in the journal Molecular Therapy has shown that the treatment prevents or delays amputation, slows down tumors and metastasis growth and improves survival.
The results show that the “OST-HER2” treatment is a less aggressive and more targeted approach to treating this invasive cancer.
In a statement released after the release of the experimental data, Romans said: “My dream has been that” Ost-Her2 “has potentially able to change the standard of bone cancer care and potentially restrict the need for amputation or the initial tumor removal with surgery.
“With today’s data, we believe that we are taking the initial steps to do so, because our comparative oncology approach as a result of the 5 % genetic identity between human and dog bone cancer makes us believe that there is a significant potential for translating this data into human beings,” he said.
Bone cancer, like dogs, is the most common primary cancer in humans, and like dogs can occur in any bone in humans, but it is more likely to form in long bones.
In January 2025, Ost reported the results of Phase 2’s human clinical tests with “Ost-HER2” for bone cancer that were extended to the lungs and were fully surgically removed.
Participants were between 12 and 39 years old. This treatment created a significant statistical result in 12 -month survival without EFS event. The EFS event is defined as a recurrence and return of metastatic bone cancer.
In addition, the “Ost-HER2” method had a positive and significant effect on overall survival.
The findings of this study have not yet been reviewed or published.
Researchers in the study say we are very pleased with these results of our Phase 2 clinical trial, as they show that patients treated with “Ost-Her2” have achieved the primary end of the 12-month recurrence.
According to them, the strong safety index shown in the study also supports the use of “Ost-Her2” in a population that currently has no approved treatment.
This new treatment as a treatment for bone cancer in children has received US Food and Drug Administration approval (FDA) and European Drug Agency (EMA)
The end of the message
(tagstotranslate) Bone Cancer (T) Cancer Treatment
RCO NEWS