The world’s first treatment to reverse spinal cord injury entered the human test.
According to RCO News Agency, A paradigm change in the treatment of spine injuries is currently in the landscape with the world’s first revival cell therapy.
According to the New Atlantic, the test has been approved for a clinical trial of the first stage of registration, which is a historical turning point that can successfully treat conditions that have so far been an untreated disease.
Recently, the US Food and Drug Administration (FDA) and the National Bureau of Medical Products (NMPA) have confirmed a global clinical test for the treatment of spinal cord injury (SCI), which is estimated to affect more than 15 million people worldwide.
Spinal cord injury affects people throughout the community and is often caused by traffic accidents, sports injuries and other injuries, including serious crashes and workplace accidents. There is no real treatment for it, and its treatment is mostly done by managing conditions, surgery and rehabilitation to restore a degree of quality of life. However, patients often grapple with limb paralysis or severe lifelong disability.
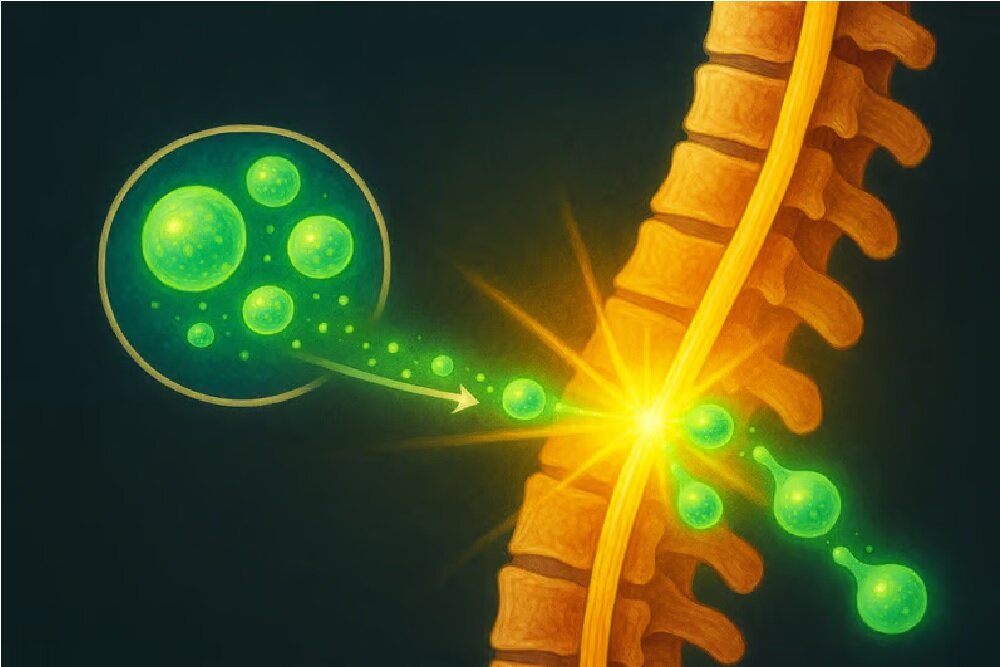
Now, Xellsmart Chinese Biotechnology Company has the potential to change the situation permanently, as it has been shown to restore the restorative treatment of alignic induction stem cells (IPSC) by US and Chinese health agencies to enter a green light clinical test.
The powerful stem cells are immature stem cells that can be converted to certain cells, in which case is an alternative to damaged or dead nerve cells caused by spinal cord injury.
The purpose of this treatment is not only a repair of injury, but also the basis for the re -growth of all cells needed to return the function to the affected area.
Every year, China and the United States report approximately 100,18,000 new cases of acute spinal cord injury, which is approximately 10 and 2 new cases per hour, respectively. Most patients experience permanent disabilities that endanger their quality of life. Due to the limited reconstruction capacity of the central nervous system, the nerve repair after spinal cord injury is very challenging.
The news of the beginning of this human trial is published after four years of pre -printing research, which aims to be widely used that does not need to harvest the cell from the patient and offers a suitable treatment for everyone that works for anyone with spinal cord injury. This means that if it goes through the experimental process, the construction and increase of the scale of this treatment will be easy for widespread access.
Also, because of the well -examined cell subgroup, allogenic cells (those that are derived from other sources and are not from the patient themselves) must have a low footprint.
This trial is done with the participation of Sun Yat-Sen Hospital in China, which is in the treatment and research on these complex spine injuries.
The experiment is the latest test for Xellsmart, which is currently testing specific cell therapy to treat Parkinson’s and ALS.
If this trial is successful and this new treatment is marketed, it will have significant potential that will include recovering performance for individuals.
The first phase of this experiment, which provides safety and effectiveness as well as dose parameters, will be completed by next year and will be transferred to the next stage if successful, which includes more population.
The second phase is then expected to begin in 2028 at the earliest opportunity, but this treatment can then be mass -produced and available within five to seven years.
“We move beyond care and treatment,” says a spokesman for Xellsmart. For the first time, we have hoped for the millions of people living with spinal cord injury.
The end of the message
(tagstotranslate) spinal cord injury (T) Treatment of spinal cord injury
RCO NEWS