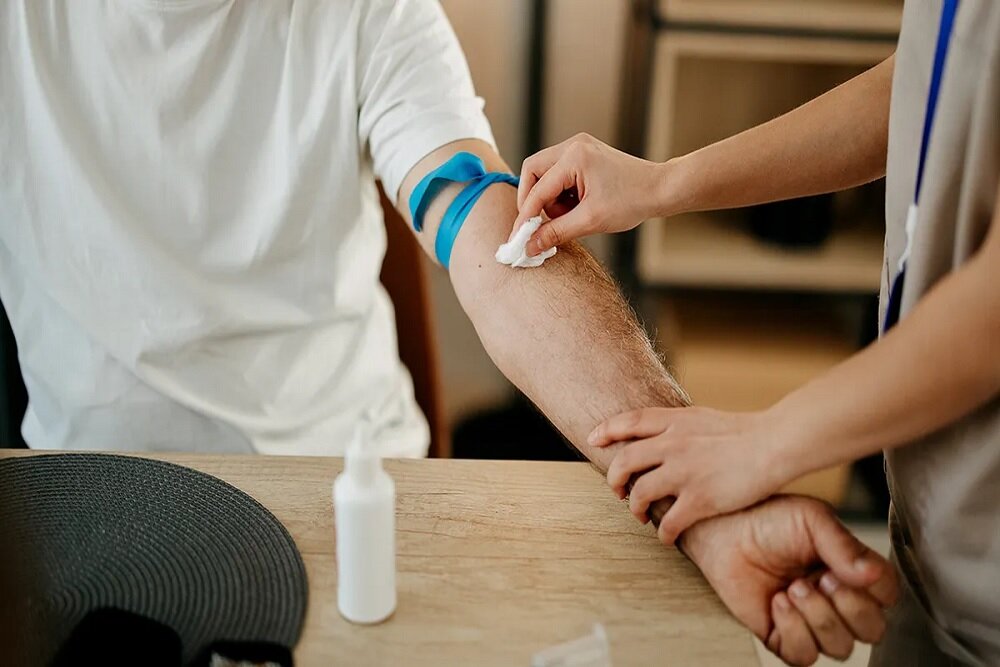
The US Food and Drug Administration (FDA) confirmed the first blood test to diagnose Alzheimer’s disease. Previously, screening the disease needed PET scan or spine sampling, which are more aggressive.
According to RCO News Agency, There is now a new method for screening and early diagnosis of Alzheimer’s disease; Because the US Food and Drug Administration (FDA) recently confirmed the first blood test to diagnose the disease.
According to Reuters, the experiment called Lumipulse was developed by Fujirebio Diagnostics and measures the ratio of two proteins related to or absence of Alzheimer’s.
Previously, screening of Alzheimer’s suspected patients was limited to more aggressive options including PET scan or spine sampling.
Lumposal testing is intended for use in clinical environments with patients showing cognitive deterioration symptoms. Of course, at least in the current form, it is not something that the general public wants as a standard screening method.
The experiment is performed by measuring two proteins “PTAU2” and “β-AMYLOD 1-4”. The blood test calculates their ratio, which is associated with the accumulation of amyloid plaques in the brain.
People with Alzheimer’s, “PTAU2” have a higher and “β-Amyloid 1-2”.
In a clinical study, the experiment identified negative results better than positive results. As such, the experiment is likely to be used to reject Alzheimer’s.
More than 5 % of the negative results correspond to the negative PET scan or other tests.
The results were a bit wrong for the positive and the success rate was 4.9 %. Therefore, the researchers say positive results should be confirmed by more advanced diagnostic tests.
The end of the message
(tagstotranslate) Blood test (T) Alzheimer’s
RCO NEWS