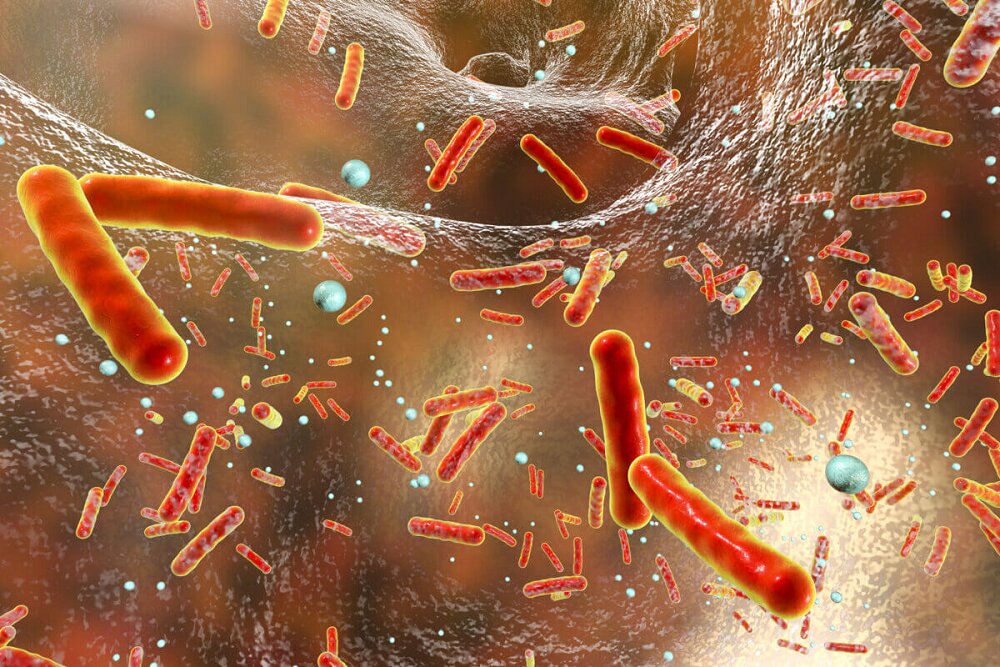
In the ongoing battle against antibiotic-resistant superbugs, researchers have discovered an unexpected vulnerability that could change the way we fight these deadly infections.
According to RCO News Agency, This discovery came at a critical time. Current estimates show a grim picture. Drug-resistant infections currently kill more than one million people annually, and it is predicted that by 2050, the number of deaths will almost double to two million per year.
However, a team led by researchers at the University of California-San Diego may have found a new way to tackle this crisis without relying on traditional antibiotics. Their research, published in the journal Science Advances, shows that antibiotic-resistant bacteria have an inherent weakness. A weakness that could explain why these seemingly unstoppable superbugs haven’t completely taken over us.
Professor Gürol Süel from the Department of Molecular Biology at UC San Diego says: “We discovered an Achilles heel in antibiotic-resistant bacteria.” We can use it to suppress the development of antibiotic resistance without using drugs or harmful chemicals.
The group studied a common bacterium, Bacillus subtilis, and focused on why antibiotic-resistant strains do not always dominate their non-resistant counterparts. What they found was surprising. The same mutations that make bacteria resistant to antibiotics also create unexpected weaknesses.
Think of it as a tug of war inside a bacterial cell. The researchers discovered that in the resistant bacteria, essential cellular components called ribosomes that help build proteins are unusually greedy for magnesium, a vital mineral. This causes internal competition with adenosine triphosphate molecules, which also require magnesium to function. This is like two critical workers competing over limited resources, ultimately making resistant bacteria less efficient at growing and spreading.
This finding is exciting because it suggests a new way to fight resistant bacteria without using traditional antibiotics. Scientists may be able to target this weakness by manipulating magnesium levels in bacterial environments, potentially stopping resistant strains while not harming beneficial bacteria.
The discovery is part of a broader effort to find non-drug alternatives to fight bacterial infections. In a separate study published last October, Sowell and his colleagues also developed a bioelectronic device that harnesses the electrical activity of bacteria to fight infections, successfully reducing the harmful effects of a common nosocomial infection.
“We’re running out of effective antibiotics, and their overuse over decades has led to the spread of antibiotics around the world, from the Arctic to the oceans and groundwater,” says Sowell. Non-pharmacological alternatives to the treatment of bacterial infections are needed, and our two recent studies show how we can achieve non-pharmacological control of antibiotic-resistant bacteria.
This development brings hope back to what has become one of the most important challenges of modern medicine. By understanding and exploiting these natural vulnerabilities, researchers may have found a new way to change the way we fight antibiotic resistance without adding more antibiotics to our already saturated environment.
end of message
RCO NEWS